This post discusses how to give the proper dose of medicine to your child as well as how Children’s Mercy Hospital is researching more in depth ways on how to determine drug dosing for children. This post is sponsored by Children’s Mercy, I am part of the Children’s Mercy Moms Team!
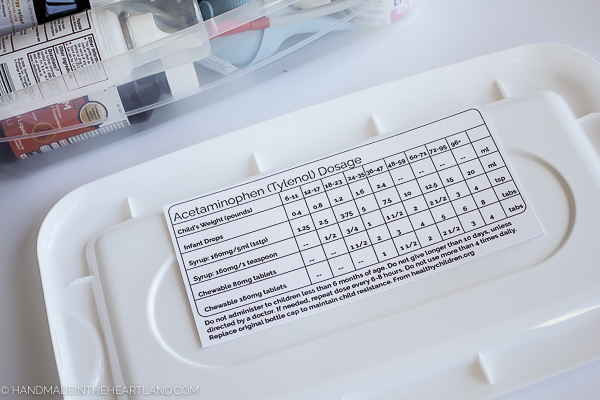
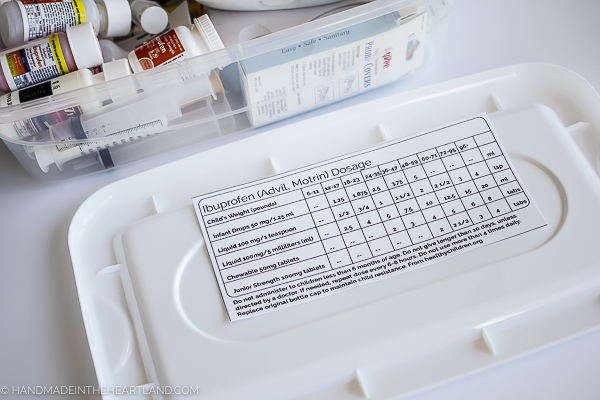
It’s almost inevitable that someone in our home is going to come down with a bug, a fever, a cough or a cold during the winter months. I have at times found it somewhat confusing on what dose of over the counter medicine to give to my kids. Until my pediatrician gave me a detailed dosing guide I was going off the the package instructions, which at times were on the box that I had thrown away!
Currently, Children’s Mercy is exploring a new platform, aptly called GOLDILOKs, that helps doctors find the right dose for treating leukemia, ADHD and other medical diagnoses. The platform integrates patients’ medical records to provide real-time results, allowing physicians to simulate a dose based on the desired effect for the patient. This model is being applied to studying cardiology drugs, asthma histamine treatment and more.
As Steven Leeder, director of clinical pharmacology and therapeutic innovation for Children’s Mercy, says, “(We’re) interested in making medications more effective for kids. People try to scale adult data for kids. … How would you scale a dose from a 70-kilogram adult to a 1-year-old?”
Additionally, physicians at Children’s Mercy are involved in the American Academy of Pediatrics Committee on Drugs and work closely with the FDA, NIH and CDC in issues related to drugs in children. The Committee on Drugs reviews all aspects of pediatric pharmacology including drug indications, contraindications, absorption rates, routes of administration, dosing, use of precautions and mode of action as they apply to children. The Committee also advises the Board of Directors in matters related to drug labeling, safety and efficacy for both prescription and over-the-counter drugs monitors federal legislation related to the drug approval process and promotes the need for expanded pediatric drug trials.